Hyperchem can perform conformational searches in which a number of torsional angles in a molecule are varied in order to locate low-energy conformations. Starting from a given structure, the torsional angles are perturbed randomly; local energy minimizations are performed for each set of trial torsional angles. Once new conformations are found, they are also used as starting points for perturbations. A running tally of different conformers (energy-minimized structures) is listed in order of increasing energy.
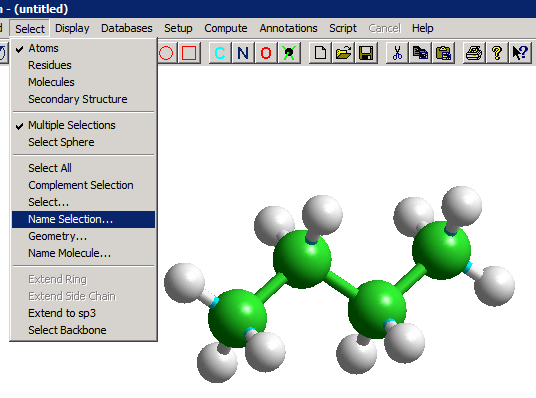
We will illustrate this for a very simple molecule, n-butane, for which only a single torsional angle can be meaningfully varied. First, we create the butane molecule, and select the four C atoms; we need to use "Name Selection" (as shown below) to provide a name (say, "Tor1") for the torsional angle between C2 and C3.

We then select "Compute" "Conformational Search". The main requirement is to define the angle(s) to be varied during the search.
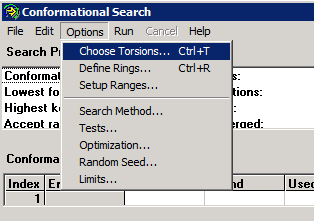
For this case, only "Tor1" can be varied. It is strongly desirable to set non-default values for the search limits - e.g.,

Once we are ready, we need to "Run" the conformational search, which for n-butane quickly converges to the three conformers one expects, with torsional angles 180 (trans), +65.1, and -65.1 degrees:
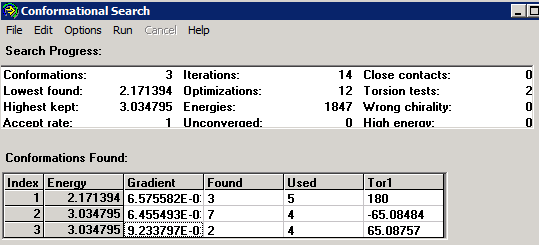
Note that the "Found" and "Used" columns indicate how many times the specific conformation was found, and how many times is was used as starting point for the conformational search. For simple molecules, generating more trials does not lead to more conformations!
The +65 and -65 degree conformers are mirror images of each other and have identical energies. A specific conformer can be selected from the table giving their energies and displayed by using "Edit" "Put Molecule" (or simply CTRL-U) - e.g., the three n-butane conformers are shown below.
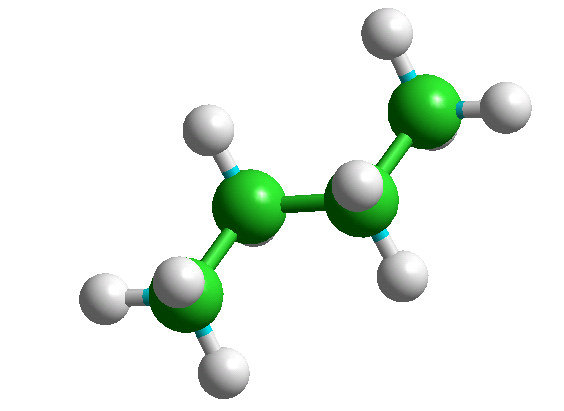 Index = 1, Tor1 = 180
Index = 1, Tor1 = 180
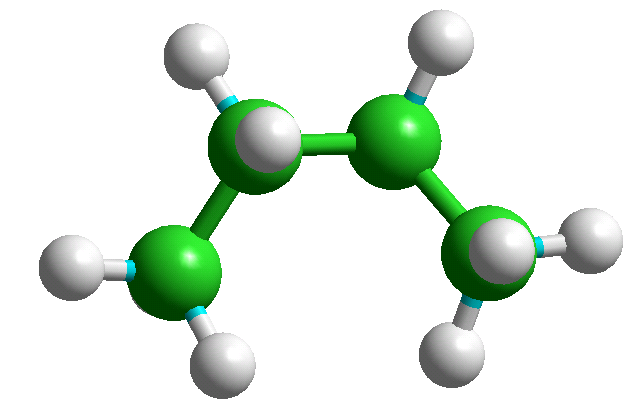 Index = 2, Tor1 = -65.1
Index = 2, Tor1 = -65.1
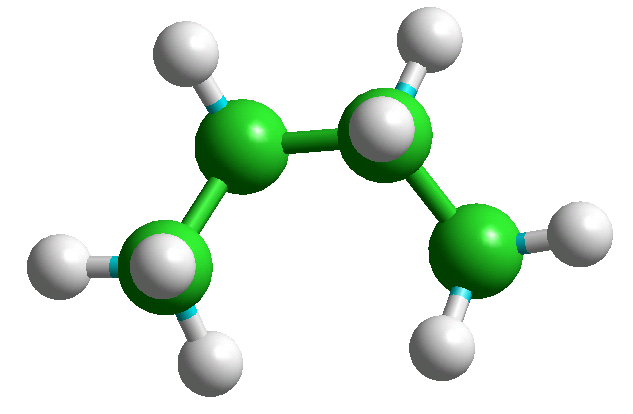 Index = 3, Tor1 = 65.1
Index = 3, Tor1 = 65.1
The contributions of each conformer to the properties are governed by Boltzmann statistics, with the weight of conformer i proportional to exp(-Ui /kBT). For example, the three conformers for butane (which differ by 0.864 kcal/mol in energy) contribute 68% (trans) and 16% each (other two) at room temperature (T = 300 K).