 Gibbs Project
Gibbs Project Gibbs Project
Gibbs Project
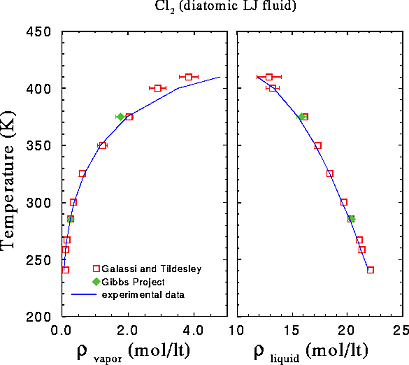 We have examined
if our program reproduces the Gibbs ensemble results of Galassi and Tildesley
(1994, Molecular Simulation, 13, 11-24) for clorine. In this
work, clorine is modelled as a molecule composed of two LJ beads. The center-to-center
distance is 0.630 sigma.
We have examined
if our program reproduces the Gibbs ensemble results of Galassi and Tildesley
(1994, Molecular Simulation, 13, 11-24) for clorine. In this
work, clorine is modelled as a molecule composed of two LJ beads. The center-to-center
distance is 0.630 sigma.
More specifically, we reproduced two points on the phase enevelope of clorine
(table2, page 15 of the arorementioned paper), at 375 and 285.2 Kelvin.
The results
of Galassi and Tildesley are given as open squares, while our
results are given as filled rhombes. Our results are the same with
those of Galassi and Tildesley within the statistical error. We calculate
the statistical error of the simulation as the (sample) standard deviation
of (five) block averages for the density. Next, we give the control-plots
for the runs performed.
This temperature is rather high. The run was without problems. You can
look at the input
file, the results
file or at the report
file, which is shorter and contains the block averages of density (at
the end). The control graphs are the following:
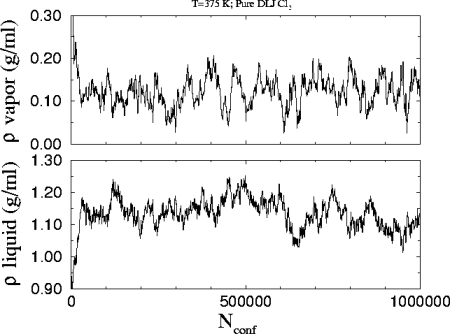 This is the
plot of the number density versus the number of attempted configurations
in the run. The starting densities are 0.99 and 0.25 g/ml for the liquid
and the vapor respectively.
This is the
plot of the number density versus the number of attempted configurations
in the run. The starting densities are 0.99 and 0.25 g/ml for the liquid
and the vapor respectively.
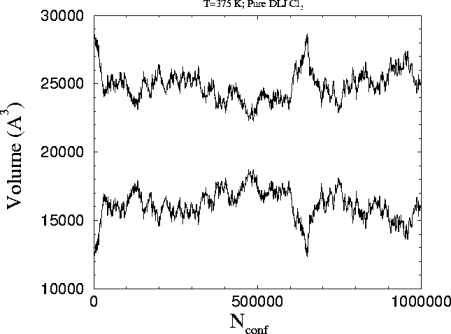 This is the
plot of the volume versus the number of attempted configurations in the
run. The starting volumes are 26877 and 14128 A3 for the liquid
and the vapor respectively.
This is the
plot of the volume versus the number of attempted configurations in the
run. The starting volumes are 26877 and 14128 A3 for the liquid
and the vapor respectively.
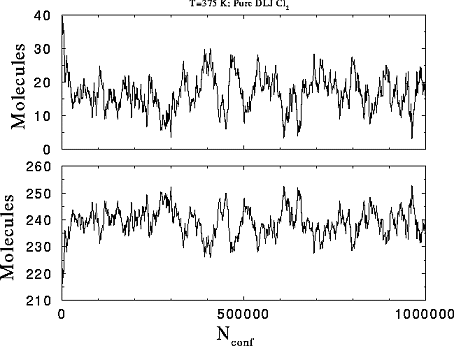 This is the plot
of the number of molecules in each phase versus the number of attempted
configurations in the run. The starting molecules are 226 and 30 for the
liquid and the vapor respectively.
This is the plot
of the number of molecules in each phase versus the number of attempted
configurations in the run. The starting molecules are 226 and 30 for the
liquid and the vapor respectively.
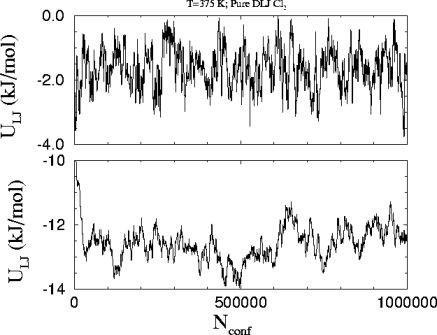 This is the
plot of the Lennard-Jones energy in each phase versus the number of attempted
configurations in the run. The bond energies are not taken into account.
This is the
plot of the Lennard-Jones energy in each phase versus the number of attempted
configurations in the run. The bond energies are not taken into account.
This temperature is low and subsequently there were difficulties in
transfering molecules from one phase to the other. In total, 6 million
trial moves were attempted with (about) 1200 molecule transfers per phase.
Two runs were pewrformed, the first with 1 million and the second with
5 million attempted steps. The second run was a continuation of the first
one. You might look at the input files of both the first
or the second
run. Of cource, the results file of the first
or the second
run are available. Better still, look at the report files or either the
first
or the second
run; they are shorter and contain the block averages of density (at the
end). The control graphs are the following:
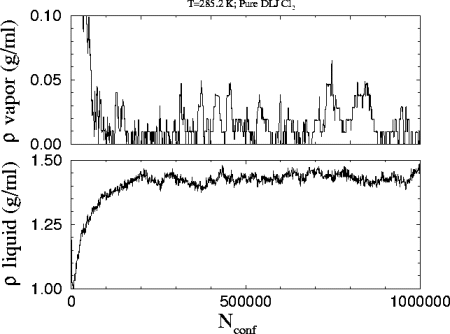 This is the
plot of the number density versus the number of attempted configurations
in the run. The starting densities were 1.24 and 0.217 g/ml for the liquid
and the vapor respectively.
This is the
plot of the number density versus the number of attempted configurations
in the run. The starting densities were 1.24 and 0.217 g/ml for the liquid
and the vapor respectively.
You can also look at the same type of graph for the second
run.
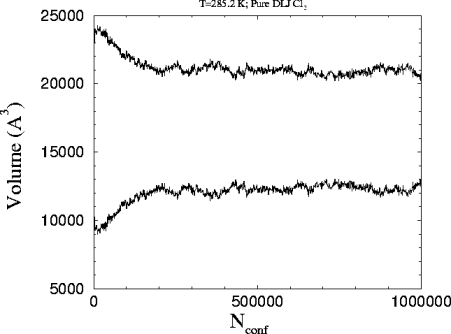 This is the
plot of the volume versus the number of attempted configurations in the
run. The starting volumes are 22407 and 10851 A3 for the liquid
and the vapor respectively.
This is the
plot of the volume versus the number of attempted configurations in the
run. The starting volumes are 22407 and 10851 A3 for the liquid
and the vapor respectively.
You can also look at the same type of graph for the second
run.
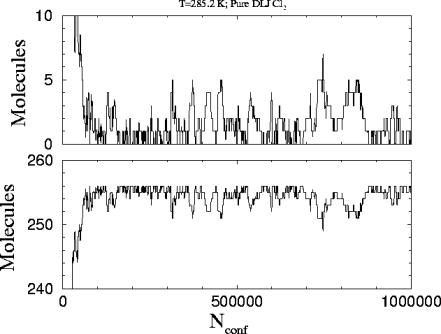 This is the plot
of the number of molecules in each phase versus the number of attempted
configurations in the run. The starting molecules are 236 and 20 for the
liquid and the vapor respectively.
This is the plot
of the number of molecules in each phase versus the number of attempted
configurations in the run. The starting molecules are 236 and 20 for the
liquid and the vapor respectively.
You can also look at the same type of graph for the second
run.
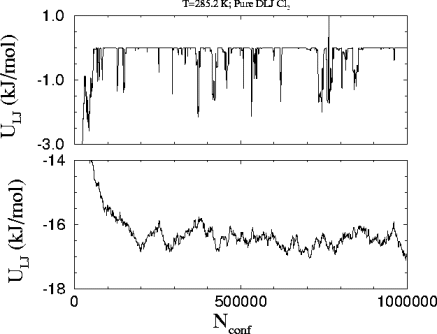 This is the
plot of the Lennard-Jones energy in each phase versus the number of attempted
configurations in the run. The bond energies are not taken into account.
This is the
plot of the Lennard-Jones energy in each phase versus the number of attempted
configurations in the run. The bond energies are not taken into account.
You can also look at the same type of graph for the second
run.
|
Go to: |
|
|---|---|